TMS Therapy
NeuroStar uses transcranial magnetic stimulation (TMS) which is FDA approved (for Major Depressive Disorder), to target key areas of the brain that are underactive in people with depression. It is not ECT (electroconvulsive therapy).
Depression is thought to be caused by an imbalance of the brain’s neurotransmitters, which are chemical messengers that send signals between the brain cells.

What is NeuroStar Advanced Therapy (TMS)?
During a NeuroStar treatment session, a magnet similar in strength to that used in a magnetic resonance imaging machine is used to stimulate nerve cells in the area of the brain thought to control mood. These magnetic pulses may have a positive effect on the brain’s neurotransmitter levels, making long-term remission possible.
Treatment with Advanced Therapy is easy
- Therapy sessions are conducted in our office
- You can return to normal activities right away
- You are awake during treatment
- There are no negative effects on memory or sleep
- It is covered by most health insurance plans, including Medicare, Tricare, and MVP
Take the first step
to overcoming your depression today
Find out whether NeuroStar TMS treatment is right for you!
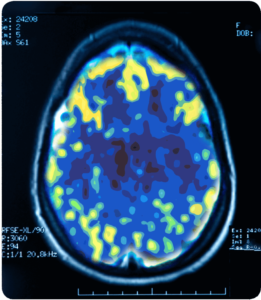
Non-Depressed
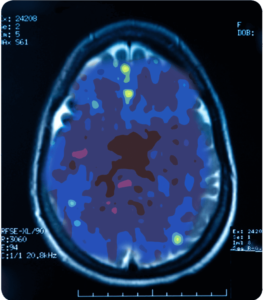
Depressed
A PET scan measures vital functions such as blood flow, oxygen use and blood sugar (glucose) metabolism.
Source: Mark George, M.D. Biological Psychiarty Branch Division of Intramural Research Programs, NIMH 1993
How NeuroStar®TMS Therapy Works
Before Treatment
You’ll recline comfortably in the treatment chair. A small, curved magnetic coil will be positioned lightly on your head.
During Treatment
NeuroStar delivers focused magnetic stimulation directly to the target areas of the brain. You’ll hear a clicking sound and feel a tapping sensation on your head.
After Treatment
NeuroStar Advanced Therapy: Depending on your doctor’s recommendation, each treatment takes between 19 and 37 minutes.
How does NeuroStar work for people with depression?
NeuroStar®TMS Patient Stories
Frequently Asked Questions
What is Transcranial Magnetic Stimulation?
How does TMS work?
How long is TMS treatment?
Is TMS Therapy covered by my insurance?
Is TMS Therapy a good alternative for patients who cannot tolerate the side effects of antidepressant medications?
Is TMS Therapy like other alternative therapies that use magnets to treat some illnesses?
Clinical Trials and Academic Studies
Carpenter LL, et al. (2012).
Transcranial Magnetic Stimulation (TMS) for Major Depression: a Multisite, Naturalistic, Observational Study of Acute Treatment Outcomes in Clinical Practice. Depression and Anxiety, 29(7):587-596. www.ncbi.nlm.nih.gov/pubmed/22689344
George MS, et al. (2010).
Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder: A Sham-Controlled Randomized Trial. Arch Gen Psychiatry, 67(5):507-516. www.ncbi.nlm.nih.gov/pubmed/20439832
Dunner DL, et al. (2014).
A Multisite, Naturalistic, Observational Study of Transcranial Magnetic Stimulation (TMS) for Patients with Pharmacoresistant Major Depressive Disorder: Durability of Benefit Over a 1-Year Follow-Up Period. J Clin Psychiatry. 75(12):1394-1401. www.ncbi.nlm.nih.gov/pubmed/25271871
O’Reardon JP, et al. (2007).
Efficacy and safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled trial. Biol Psychiatry, 62(11):1208-1216. www.ncbi.nlm.nih.gov/pubmed/17573044



